
In our business we spend a lot of time working out how to keep things cool.
This often involves using frozen gel or water.
The gel or water is typically contained in polythene sachets (as shown) and is frozen down to −20ºC before being placed around the goods to be protected from high temperatures.
As significant mounts of energy is required to increase the temperature of the ice and convert it to water, we can reduce the amount of energy reaching the ice, by covering the goods with a thermal foil cover that reflects the thermal energy the ice needs to melt. This maintains low temperatures for longer by slowing the transfer of heat keeping products cool for longer.
Water is one of the few chemical substances on earth that exists in the solid, liquid and gas states at normal temperatures.
Phase change
Large amounts of energy are needed to change the phase of water, from solid to liquid (ice to water), and liquid to gas (water to steam).
This energy is called latent heat.
It is latent or hidden, because in phase changes, energy enters or leaves a system without causing a temperature change in the system; so, in effect, the energy is hidden.
So while ice is melting, its temperature does NOT increase. It remains at 0ºC until all the ice has melted. And it requires a lot of heat energy to melt.
334 kJ is the energy needed to melt a kilogram of ice. This is significantly more than other substances. This is a lot of energy as it represents the same amount of energy needed to raise the temperature of 1 kg of liquid water from 0ºC to 79.8ºC. Even more energy is required to vaporise water; This shows that the energy required for a phase change is enormous compared to energy associated with temperature changes without a phase change.
The graph below shows the effects of phase change by looking at the effect of adding heat into a sample of ice at −20ºC.
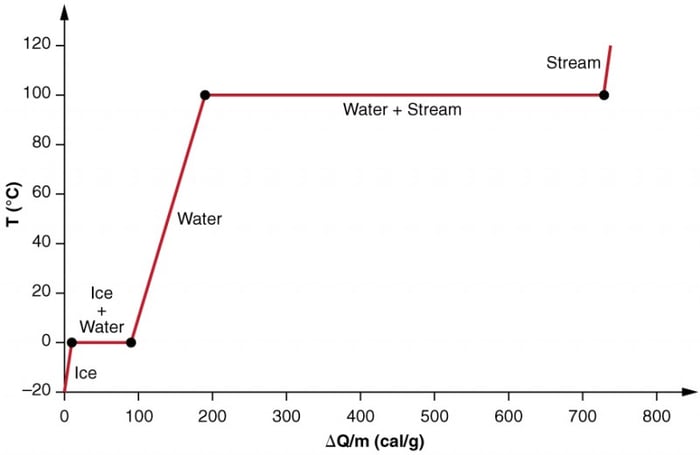
From −20ºC to 0ºC the temperature increases linearly absorbing heat at a constant rate of 0.50 cal/g⋅ºC.
Once at this temperature, the ice begins to melt until all the ice has melted, absorbing 79.8 cal/g of heat.
The temperature remains constant at 0ºC during this phase change.
Once all the ice has melted, the temperature of the liquid water rises, absorbing heat at a new constant rate of 1.00 cal/g⋅ºC.
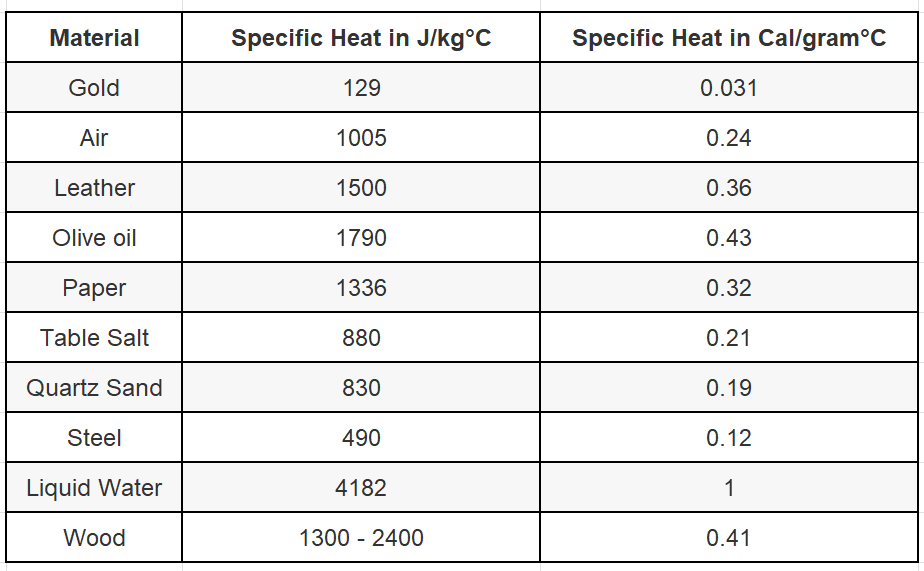
The specific heat capacity of water
Specific heat is a measure of heat capacity, or how much heat a material can store when changing temperature. A high heat capacity means that a substance can absorb a lot of heat before registering a change in temperature.
This article explains why water has such a high specific heat capacity. It explains it very clearly and concisely.
This table is taken from this article.
A real life example
An air freight customer of our using gel ice packs with foil covers, had a consignment delayed in Qatar. The goods had to be kept below 8ºC.
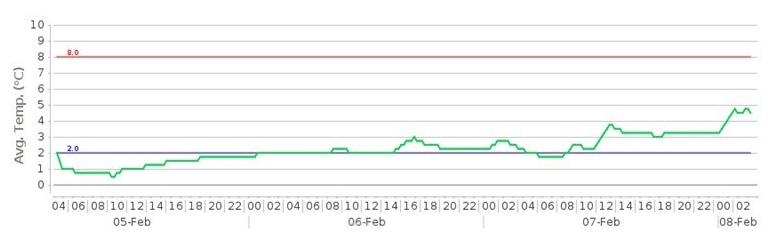
Without any external refrigeration facilities, the goods were delivered in a satisfactory condition despite the high temperatures it was exposed to.
This demonstrates the effective combination of using ice packs with a reflective foil cover to maintain the desired temperatures over three days.
To find out about our thermal protective products you can visit our website here.



