This article aims to help people working in cold chain logistics understand the
transfer of thermal energy (heat) and is an attempt to explain as simply as possible
the science behind keeping things cold.
‘Heat’ is the main environmental hazard in the transportation of temperature sensitive goods.
There are a variety of protective packaging products that will aid temperature control, and refrigerants to maintain low temperatures. Some of these are discussed in this article.
The need of temperature control
There are many products that will rendered unusable if exposed to unfavourable
temperatures beyond a certain time limit. These include perishables,
pharmaceuticals, biological products, frozen food, fresh fruit and vegetables, fish,
meat and dairy products.

The challenge facing the logistics industry is transporting these valuable commodities without deterioration so they arrive in good condition and in a usable state.
Road and sea transport can utilise refrigerated transport for some temperature sensitive items, but many listed above will not survive a journey of several weeks, and other products such as vaccines need to spend minimal time in transit where there is a risk of compromising their usability, so are usually transported via air freight.
Transport via air freight requires temperature sensitive goods to be packed so they
are not dependent on external work (e.g. refrigeration) to maintain their desired
temperatures. The temperature in an airplane cargo hold can fluctuate, and there
can be delays loading and unloading, with goods sat on the tarmac in direct
sunlight.

The science of thermal dynamics
This article attempts to explain the science of ‘thermal dynamics’ which explains how ‘heat’ or ‘thermal energy’ transfers or flows from one object to another. This may help with decision making when trying to work out what thermal protection solution is best suited to protect your chilled and frozen goods in transit.
What is 'heat'?
Heat is energy in transfer to or from a 'thermodynamic system'
Note the word 'transfer'.
What is a thermodynamic system?
A Thermodynamic System is a part of the physical universe with a specific limit for
observation. Real or imaginary walls can define this limit.
As an example, a ‘thermodynamic system’ could be a pallet load of temperature sensitive products.
First, how does heat behave?
The ‘Second Law of Thermodynamics’ states that the flow of thermal energy which
we call “heat” goes from hot to cold every time until the temperatures are equalised.
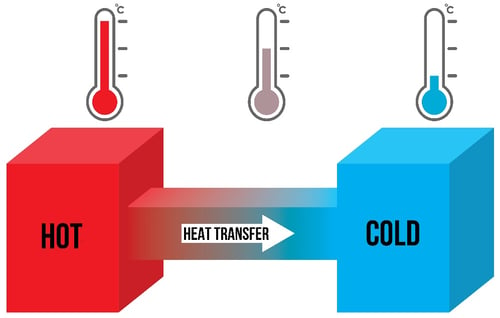
In the same way that water flows from high to low, heat flows from hot to cold.

So, to keep cold items cold, we have to try and stop the flow, or transfer, of heat
from hot to cold.
How does thermal energy (or heat) transfer?
Heat transfers or flows, by three processes.
• CONVECTION,
• CONDUCTION
• RADIATION
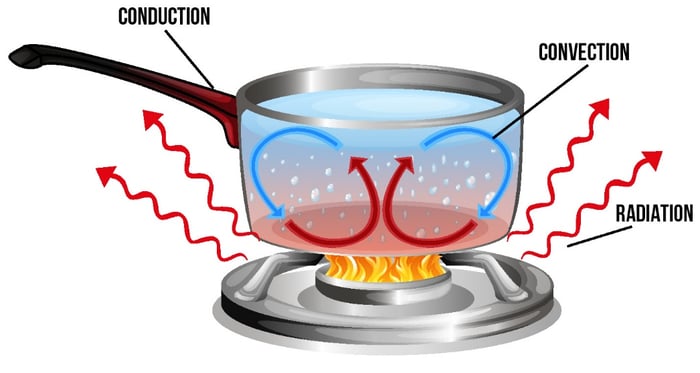
CONVECTION is the heat transfer due to the movement of a fluid, such as a gas or liquid. Hot gas or liquids will always rise and move up and cold gas or liquid takes its place.
CONDUCTION is the transfer of heat between substances that are in direct contact
with each other. The better the conductor, the more rapidly heat will be transferred.
Metal is a good conduction of heat (and is known as a thermal conductor)
Air and water are poor conductors of heat and hence are known as thermal
insulators.
RADIATION - Heat can be transferred by radiation. Unlike conduction and
convection - which need particles - infrared radiation is a type of electromagnetic
radiation that involves waves.
Because no particles are involved, radiation can flow through the vacuum of space.
This is why we can still feel the heat of the Sun even though it is 150 million km away
from the Earth.
How does this knowledge help us to protect temperature sensitive goods in transit?

So, in this theoretical example, we have a thermodynamic system, a pallet of boxes which have chilled goods inside.
The cardboard boxes are made from corrugated paper which is a poor conductor of heat. However, radiation from direct sunlight will heat the boxes up, which will eventually heat up the air contained in the boxes, which will heat up the chilled goods through convection.
Therefore we need to find methods that will interfere with the transfer of thermal
energy (heat) to the chilled products.
Here are some examples, and some explanations how they interrupt the flow of
heat.
Temperature control solutions
Thermal quilted pallet cover
This relies on natural man-made fibres creating an air filled void between two layers of fabric.
Convection is transfer of heat when a fluid or gas is in motion. Since air and water do not readily conduct heat, they transfer heat through their motion.
Insulation from heat transfer by convection is usually done by either preventing the motion of the fluid or gas. Air trapped in the fibres of the quilted insulation cannot move and transmit heat by convection, and as air is a poor conductor it acts as an insulation.
 |

Air trapped by the fibre filling is a poor conductor of heat, and convection is limited due to lack of air movement. |
FOIL pallet cover
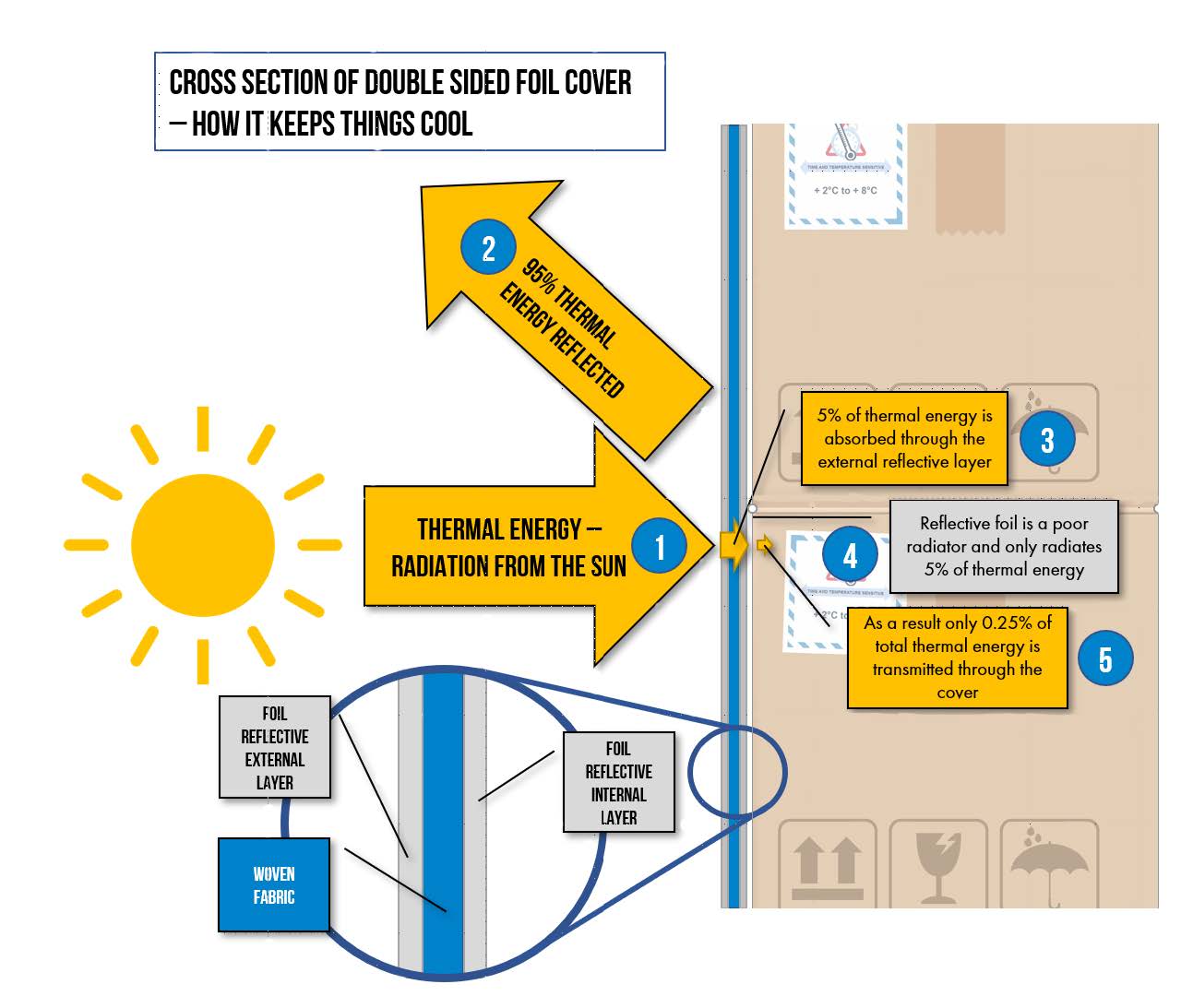
Foil pallet covers come in two main variations. Either single sided with foil on the outside, or double sided with a reflective foil surface inside and out.
Aluminium foil is one of the most reflective materials readily available, and reflects 95% of thermal energy from the sun.
Heat from the sun is transmitted by the process of radiation and the energy from light converts to heat as it is absorbed by any surface it hits.
The reflective surface reflects 95% of this energy keeping the pallet contents cool.
However, if it is a double sided foil pallet cover with a foil layer on the INSIDE, this has an additional role in reducing the transfer of thermal energy.
How is that?
This graphic below explains this.
- Infrared energy waves travelling as light from the sun (or other radiant energy
source) hit the foil pallet cover - 95% of the thermal energy is reflected
- 5% of the thermal energy is absorbed and converted to heat. A percentage of
this is conducted through the pallet cover. For this purpose we will assume all
5% reaches the inner foil surface. - The reflective inner foil surface is a poor radiator, and only emits 5% of its heat.
- Theoretically, this means that only 0.25% of the original thermal energy that hit
the outside of the foil pallet cover, reaches the product inside.
A bit more about radiation
If we consider the difference between a black object and a shiny silver object in terms of radiation emissivity.
Although the two objects may be identical, if the surface of one is covered with a material of 90% emissivity, and the surface of the other with a material of 5% emissivity, the result would be a huge difference in the rate of radiation flow from these two objects even though both objects are the same temperature.
So an ideal colour for a home central heating radiator would be black.

The diagram below shows the difference of reflection and radiation of thermal energy between a black object and a shiny object.
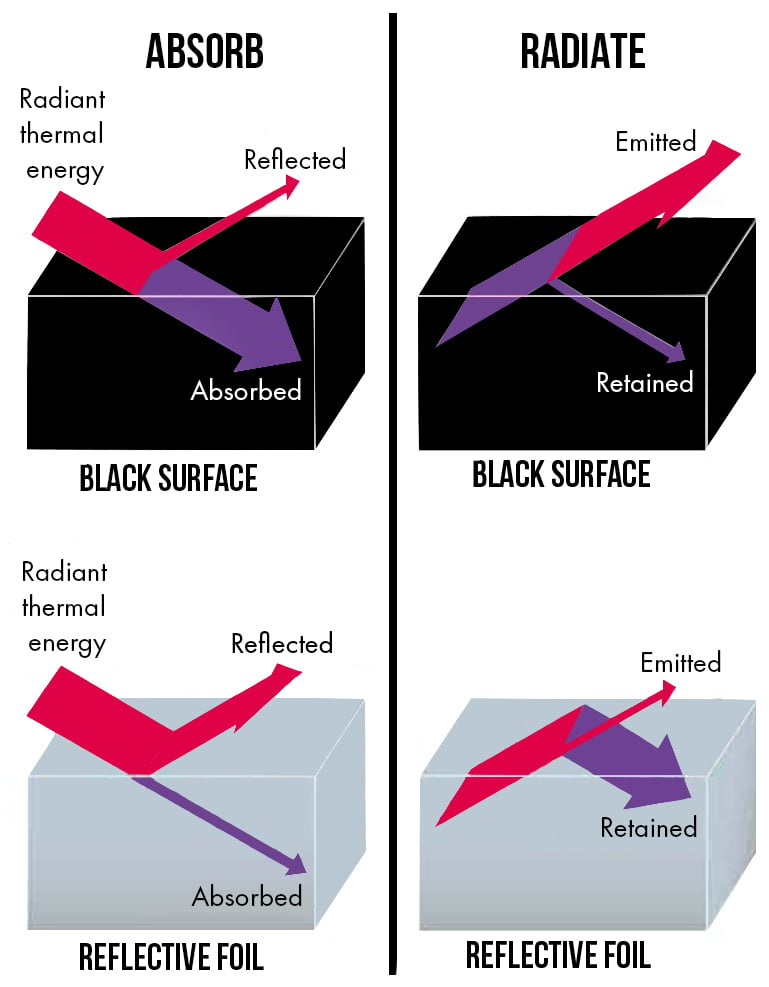
These effects are complementary. If an object has a high emissivity, it doesn't reflect.
If it has a low emissivity, it has a high reflection.
HONEYCOMB PALLETS
These are a lightweight and very strong paper based board which has a honeycomb construction that traps air inside the board.
As we mentioned before, air is a poor conductor of heat, so the trapped air insulates against the transfer of thermal energy keeping the interior cool.
 |

|
The bonus point about honeycomb board is it is 100% recyclable and biodegradable.
Gel ice packs
These are frozen before use and are placed in and around temperature sensitive products and used in conjunction with other thermal protection.
These of course will melt gradually as some thermal energy reaches inside a temperature controlled load.
 It is important to realise the no temperature change occurs during the change of state (i,e ice to water) called PHASE CHANGE.
It is important to realise the no temperature change occurs during the change of state (i,e ice to water) called PHASE CHANGE.
The temperature will only rise or fall once the change of state is 100% complete. (i.e. ALL the ice has melted)
The diagram below shows how the temperature of a gel ice pack will change with time due toe steady heat input (external heat loss has been ignored for this simple illustration).
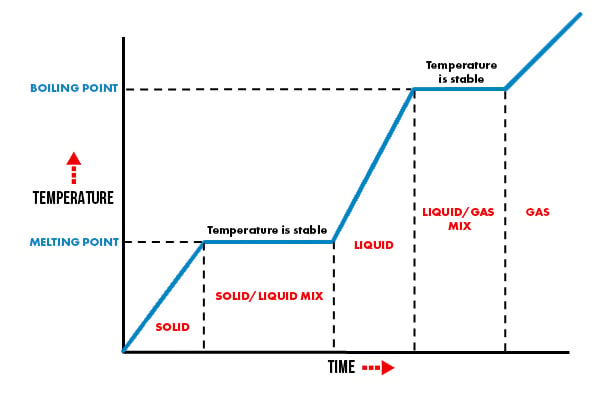 Therefore the melting gel ice packs will remain at 0°Celcius until the ‘change in state’ is complete and all ‘ice’ has changed to liquid. Only then will the temperature start to increase again.
Therefore the melting gel ice packs will remain at 0°Celcius until the ‘change in state’ is complete and all ‘ice’ has changed to liquid. Only then will the temperature start to increase again.
Latent heat
Changing the physical state of the material from solid to liquid requires the addition of heat. When energy is supplied to a solid at its melting point, the energy causes the solid to melt without changing its temperature.
During a phase change, the energy supplied goes into breaking the molecular bonds that make it a solid. Latent heat is the term used to describe the heat energy that accompanies a change of state without a corresponding change in temperature.
Different sizes and shapes of gel packs – advantages and disadvantages
The shape of the gel packs plays a significant role. With large surface and small volume, gel packs melt faster but keep the product cooler, and with a small surface and large volume, the gel packs last longer but the product is not kept as cold.
To establish which type of gel pack is best suited for different applications, then transit trials are recommended to be sure that the combinations of thermal pallet protection and ice gel packs achieve the required result.
Polystyrene sheets
Expanded polystyrene (EPS) is widely used for thermal protection including in houses. In fact, if you need a small quantity of polystyrene sheet, a builders merchant is good cheap source, and usually they hold this in stock in different thicknesses.

Calculating the best solutions
The best way, in my opinion, is to draw on the past experience of transporting temperature controlled products, and carry out transit trials with temperature monitors that ascertain the most effective temperature control solution for your particular requirement.
If you are a maths geek, you could have a stab at doing some calculations, but these will be theoretical, and will need confirmation through transit trials.
If you are interested in doing some math, click on this link to learn more about thermal transfer of energy through different materials and gases.
https://www.physicsclassroom.com/class/thermalP/Lesson-1/Rates-of-Heat-
Transfer
And finally, just for your interest
The materials listed below are all known as thermal insulators. and the smaller the number, the better the insulator. In this list AIR is the best insulator.
| MATERIAL | k |
| AIR (g) | 0.024 |
| Cotton wool (s) | 0.029 |
| Expanded polystyrene (s) | 0.03 |
| Sheeps Wool (s) | 0.038 |
| Cellulose (s) | 0.039 |
| Glass Wool (s) | 0.04 |
| Wood (s) | 0.13 |
Validation
Most suppliers of thermal control packaging will have carried out validation tests which will be available on request. These will demonstrate a products effectiveness during a range of temperature control scenarios, and will be a good reference point for working out which packing solution will work for each particular requirement.
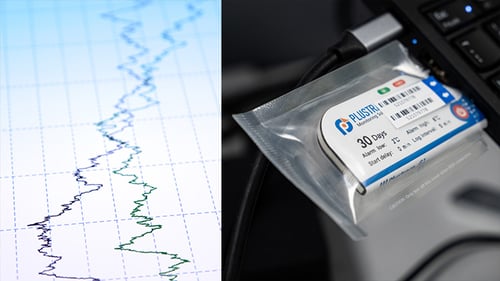
Temperature data loggers can be purchased cheaply and will monitor and log temperatures within a trial load during transit and provide data that can be analysed to confirm (or not) the suitability of a temperature control solution.

